About 30K volunteers will receive shots in tests by Moderna for a coronavirus vaccine
John Johnson
In this March 16, 2020, photo, a subject receives a shot in the first-stage safety study clinical trial of a potential vaccine by Moderna for COVID-19 at the Kaiser Permanente Washington Health Research Institute in Seattle. (AP Photo/Ted S. Warren, File)
(Newser) – The early, small-scale testing is over, and now the largest trial to date for a COVID-19 vaccine is underway, reports the AP. This one involves the vaccine made by Moderna, and the first of 30,000 volunteers in the US will begin getting shots on Monday. Half will get the real thing, half a placebo, and researchers will then begin assessing how well it keeps people from contracting the coronavirus around the US. The Moderna vaccine is the first of a handful of candidates to reach this stage—another out of Oxford University begins its large-scale trial in August. Coverage:
• Reality check: Under the best-case scenario, an initial vaccine might be available by the end of the year, but the first one to emerge might be more about reducing the severity of the virus rather than eliminating it, reports Axios. “Maybe it doesn’t prevent you from getting infected, but it prevents you from getting hospitalized, or prevents you from dying,” says a John Hopkins expert. Still, even “that would be huge.”
• Reality check, II: Another issue is that if enough people decide to skip the vaccine—and polls suggest roughly half of Americans are either leaning that way or unsure—that makes it harder for “herd immunity” to emerge, reports the Atlantic. Then there’s the staggering logistics of getting 300 million doses ready for the US, or perhaps 600 million doses if a second dose is needed, as seems likely. “Even when a vaccine is introduced, I think we will have several months of significant infection or at least risk of infection to look forward to,” says Jesse Goodman, former chief scientist at the FDA.
• Side effects: The vaccines that emerge are likely to be “reactogenic,” reports STAT News, meaning they will have side effects including headache, fever, sore arms, fatigue, and chills. For example, one of the early volunteers in the Moderna trial sought medical care after his fever spiked to 103 after his second dose. Medical experts say it’s wise to begin explaining all of this now to Americans.
• Trump message: A separate story at Axios focuses on the political aspect of this for President Trump, predicting that his new strategy will be to focus heavily on vaccines and treatments. “If he … stays on message and offers people a light at the end of the COVID tunnel, he’s gonna be in a lot stronger position to win reelection than I think a lot of people think right now,” one administration official tells the outlet.
• Warp Speed: Moderna is one of a number of companies getting support from the federal government’s Operation Warp Speed project to produce a vaccine as quickly as possible, reports CNN. The company said Sunday it received another $472 million from the Biomedical Advanced Research and Development Authority toward that end.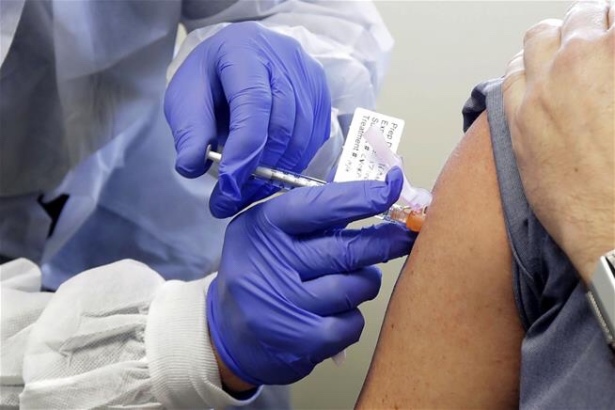
No comments:
Post a Comment